Safety Evaluation of Cosmetic Ingredients: Risk Characterisation
Ensuring the safety of cosmetic products relies on a meticulous evaluation of their ingredients, a process where risk characterisation emerges as a pivotal component.
Risk characterisation
Risk characterisation is the process of assessing and quantifying the potential risks associated with the use of cosmetic ingredients.
It usually involves an expert evaluation of the potential non-quantifiable adverse effects, followed by the calculation of a margin of safety. This calculation depends on the systemic exposure to the ingredient and its toxicological parameters.
This step determines the relationship between the dose and the likelihood and severity of adverse effects.
Margin of Safety
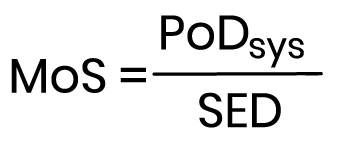
The Margin of Safety (MoS) represents the ratio between the estimated exposure to a cosmetic ingredient and the level at which adverse effects are observed. In essence, it serves as a safety buffer that allows for uncertainties in risk assessment and ensures consumer safety.
The formula for calculating the MoS is as follows:
The PoDsys is a dose descriptor for the systemic exposure to a substance, usually a NOAEL (No Observed Adverse Effect Level), and is calculated from the oral PoD (Point of Departure) by use of the proportion of the ingredient systemically absorbed. SED represents the Systemic Exposure Dose.
The calculated MoS is compared with a reference MoS, which is comparable to the uncertainty or assessment factor used in risk and safety assessments to extrapolate from a group of test animals to an average human being, and subsequently from average humans to sensitive subpopulations. A default value of 100 (10x10) accounting for inter- and intraspecies differences is generally accepted and a MoS of at least 100 therefore indicates that a cosmetic ingredient is considered safe for use.
Risk characterisation is a critical step in the safety evaluation of cosmetic ingredients. By thoroughly understanding and assessing potential risks, stakeholders can contribute to the formulation of safer and more reliable cosmetic products for consumer use.
References:
Regulation (EC) No 1223/2009 of 30 November 2009 on cosmetic products