How Cosmedesk calculates SED and MoS of cosmetic ingredients

Cosmetics legislation requires that every cosmetic product placed on the European Union market is safe to use. The safety of a cosmetic product is mainly based on the safety of the composing ingredients. A typical safety evaluation procedure includes: hazard identification, exposure assessment, dose-response assessment and risk characterisation, which involves an expert evaluation of the potential non-quantifiable adverse effects, followed by the calculation of a Margin of Safety (MoS).
The MoS is defined as the ratio between a dose descriptor for the systemic exposure to a substance and an estimate of the exposure, and it is an indicator of whether the product can be considered safe or not.
As a support tool in the safety assessment process, Cosmedesk is able to automatically calculate the Systemic Exposure Dose (SED) and the MoS for each ingredient that goes into the composition of a cosmetic product, in accordance with the SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, latest revision.
The calculation of the SED allows the estimation of exposure values of an organism to a particular substance, taking into account:
- Eproduct (mg/kg bw/day) - Estimated daily exposure to a cosmetic product per kg (body weight) based on the applied quantity and frequency of application;
- C (%) - Concentration of the substance in the finished cosmetic product;
- DAp (%) - Dermal Absorption as a percentage;
- DAa (μg/cm2) - Dermal Absorption as amount per surface, resulting from an assay under in-use mimicking conditions;
- SSA (cm2) - Skin Surface Area expected to be treated with the finished cosmetic product;
- fappl (day-1) - Frequency of application of the finished product;
- bw (kg bw) - human body weight
Accordingly, there are two ways of calculating the SED, depending on the way the dermal absorption of the substance is reported, and they are both covered by Cosmedesk:
-
- Dermal absorption of the substance reported in µg/cm²:

-
- Dermal absorption reported as a percentage of the amount of substance applied:
The MoS is then calculated using the following mathematical formula:

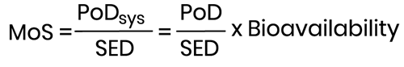
It is considered that not more than 50% of an orally administered dose is systemically available. Thus, in the absence of data, 50% of the administered dose is used as the default bioavailability value for a cosmetic ingredient and the PoDsys is derived from the PoD by dividing with a factor 2. If there is information to suggest poor oral bioavailability, a default value of 10% oral absorption could be also considered. Whenever oral absorption data are available, these should be used.
When performing the safety assessment of your cosmetic products, the safety calculations do not need to be a challenge. Talk to us and discover how Cosmedesk can help.
References:
SCCS (Scientific Committee on Consumer Safety), SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation 11th revision, 30-31 March 2021, SCCS/1628/21.
Commission Implementing Decision of 25 November 2013 on Guidelines on Annex I to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products (2013/674/EU).
![[Logo Cosmedesk]](/media/ljbbhnk4/logo-cosmedesk.svg)